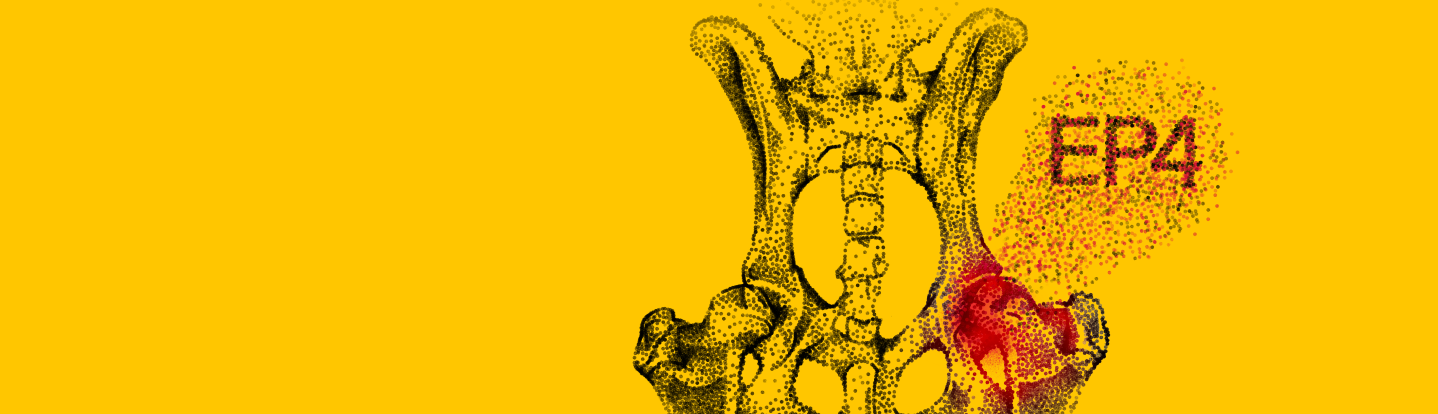
Controlling joint inflammation is critical. Galliprant® (grapiprant tablets) selectively blocks the EP4 receptor, the primary mediator of PGE2-elicited inflammation and peripheral sensitization (windup).1,2,3
Why Galliprant is a first-line choice for canine OA treatment.
 First-in-class non-COX inhibiting NSAID
First-in-class non-COX inhibiting NSAID Targets the EP4 Receptor
Targets the EP4 Receptor Use for All Stages of OA*
Use for All Stages of OA* Safe to Use Daily
Safe to Use Daily
How Galliprant Works
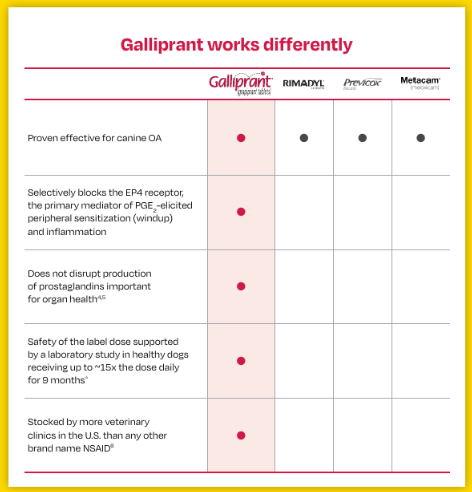
Not all NSAIDs work the same
While other NSAIDs on the market are also proven effective against canine OA, Galliprant is the only one that specifically blocks the EP4 receptor for reliable, safe relief. That’s why veterinary clinics stock Galliprant more than any other brand name NSAID.4

Proven Efficacy
- Galliprant doesn’t just mask pain; it controls inflammation and pain at the source by targeting the EP4 receptor of PGE2.
- In a real-world study, young** dogs treated with Galliprant continuously for 4 months showed significant improvements in gait analysis, overall mobility and comfort, quality of life and sleep quality.5
- Galliprant was proven effective at improving pain interference, pain severity, quality of life and veterinary assessments.6
Proven Safety
- Unique MOA reduces the impact on organ health.7,8
- Safety of label dose is supported by laboratory study in healthy dogs receiving ~15x the dose continuously for 9 months.†
Dosing & Administration
The dose for Galliprant is 0.9 mg/lb (2 mg/kg) once daily. The safe use of Galliprant has not been evaluated in dogs younger than 9 months of age.
Most dogs can be dosed with a whole or half a tablet once a day for effective canine OA pain relief (dogs weighing less than 8 lbs (3.6 kg) cannot be accurately dosed). You should prescribe Galliprant in half-tablet increments, except when using the 100mg tablet this should not be broken in half.
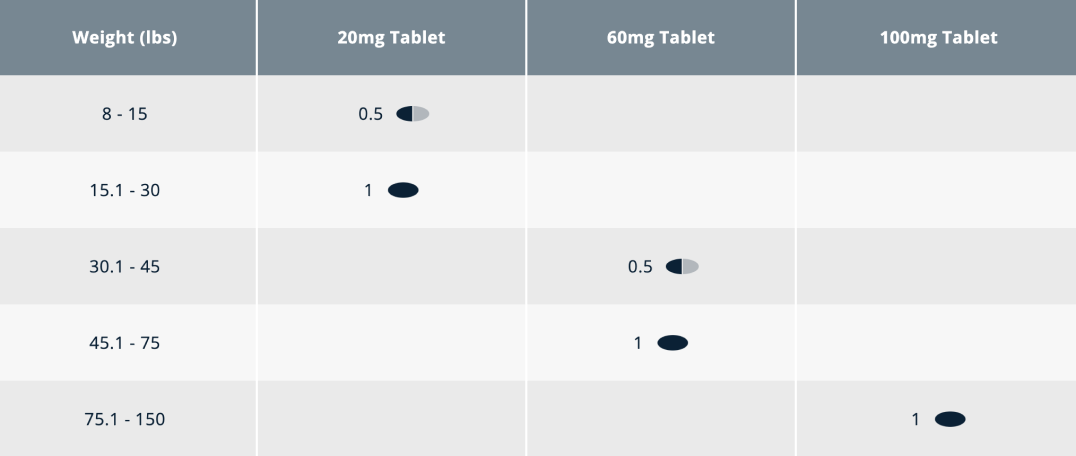
Dogs weighing less than 8 lbs (3.6 kg) cannot be accurately dosed. Only the 20 mg and 60mg tablets of Galliprant are scored. Dosages should be calculated in half-tablet increments, except when using the 100 mg tablet because it is not scored and should not be broken in half. For dogs weighing more than 150 lbs (68 kg), use a combination of tablets and half tablets to achieve the appropriate dose.
Canine OA Treatment Guidelines
For dogs in stage two of OA and beyond, experts recommend 1-3 months of daily non-steroidal anti-inflammatory drug (NSAID) therapy in newly diagnosed cases before considering tapering, with moderate to severe cases often requiring lifelong daily treatment.9,10,11

Stage 1 - Asymptomatic
No clinical signs but OA risk factors present
● Breed
● Intense activity
● Excess body weight
● Joint injury
● Joint surgery

Stage 2 - Mild OA
● Inconsistent and subtle changes in gait and posture
● Occurs with or after activities
● May appear stiff after periods of rest but appear to warm out of it

Stage 3 - Moderate OA
● Obvious changes in gait and posture
● Persistent lameness
● Some difficulty getting up or laying down
Stage 4 - Severe OA
● Severely abnormal gait and posture
● Reluctant to move
● Marked difficulty getting up, laying down or going up/down stairs
Even in their early years, there are many factors that place dogs at greater risk for canine OA
 Breed Predisposition
Breed Predisposition Intense Activity
Intense Activity Joint Injury/Surgery
Joint Injury/Surgery Obesity
Obesity
Galliprant is specifically designed to treat both inflammation and pain without tradeoffs
Dr. Medina’s Story
Dr. Medina takes her oath of caring for animals to heart. With Galliprant, she can prescribe safe and effective OA inflammation and pain relief for her canine patients.
Galliprant Resources
Indication
Galliprant is an NSAID that controls pain and inflammation associated with osteoarthritis in dogs.
Important Safety Information
Not for use in humans. For use in dogs only. Keep this and all medications out of reach of children and pets. Store out of reach of dogs and other pets in a secured location in order to prevent accidental ingestion or overdose. Do not use in dogs that have a hypersensitivity to grapiprant. If Galliprant is used long-term, appropriate monitoring is recommended. Concomitant use of Galliprant with other anti-inflammatory drugs, such as COX-inhibiting NSAIDs or corticosteroids, should be avoided. Concurrent use with other anti-inflammatory drugs or protein-bound drugs has not been studied. The safe use of Galliprant has not been evaluated in dogs younger than 9 months of age and less than 8 lbs (3.6 kg), dogs used for breeding, pregnant or lactating dogs, or dogs with cardiac disease. The most common adverse reactions were vomiting, diarrhea, decreased appetite, and lethargy. Click here for full prescribing information.